A Comprehensive and Informative Introduction to PEM Assembly
2020-10-26Learn About The Concept of PEM Assembly
PEM Assembly technology offers a breakthrough. Well as we all know there is a worldwide concern for the protection and well-being of planet Earth. Climate change is no longer a threat but actually has become an evident reality. Consequently, research has lead to finding an adequate replacement of fossil fuel energy through alternative energy sources.
Proton Exchange Membrane technology happens to be one of the most promising discoveries of the 21st century. It has helped scientists to opt for Fuel cells instead of the traditional energy source. Fuel cells are highly efficient, climate-friendly, and have low maintenance costs as well.
Although Fuel cells have gained much-required attention it is not the only use of PEM. In fact, the PEM Assembly is vital in a range of application areas such as water purification and gas separation.
PEM technology-A break through
PEM Assembly Functionality
Before we appreciate the PEM applications it is essential that we understand how the PEM functions. The Proton Exchange Membrane happens to be a semi-permeable membrane. It is usually polymer in nature but we can also come across composite membranes as well.
The PEM Assembly is maneuvered in such a magnificent way that it allows only protons to be conducted. However, at the very same time, it also acts as an electronic insulator.
This happens to be the beauty and essence of the membrane and that is why you can use it in Fuel cells and other applications. It simply separates the reactants and the transport of protons. Also, at the very same time, the PEM blocks a direct pathway through the membrane.
You categorize the PEM membranes essentially through their proton conductivity, methanol permeability, and thermal stability.
So, now that you understand how a PEM works let us move on to its applications.
PEM Fuel Cell
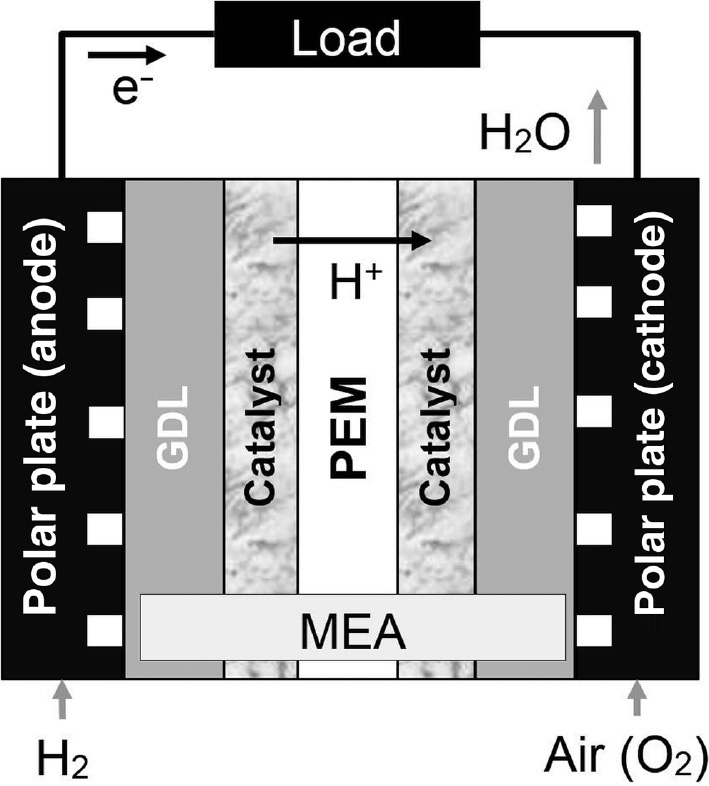
Fuel is one of the most popular and widely used applications of PEM. The membranes used in the Fuel cell have high chemical stability and act as an electrolyte as well as a separator. The separation of gases is very important as it the basic ground upon which the fuel cell works. The PEM very beautifully transports protons from the anode to the cathode and at the very same time ensures insulation.
PEMFC has out beaten solid oxide fuel cells. PEMFC’s operate at a much lower temperature and are lighter and compact in nature. This makes them an excellent source of fuel car cells.
Main Reaction in the PEMFC
Let us take a look at the main reaction taking place in a PEM Assembly fuel cell. The PEMFC uses hydrogen as its fuel. However, hydrogen oxidation by air is not the only available source. Thus, you have other options such as methanol and formic. The choice of fuel actually depends on the medium material.
Below helps us understand the PEMFC further. The PEMFC has a polymer membrane that is quite flexible in nature. The electrolyte thickness varies from 15 mm to 180mm.
Chemical Reaction in the Fuel Cell
You introduce hydrogen through the anode compartment and air goes through the opposite cathode compartment.
Both of the specific gases go through a gas diffusion layer with the help of diffusion and convection. Therefore, The gas diffusion layer is highly important in the fuel cell as it enables the reactant to be delivered and remove the actual product from the electrodes. It also makes sure that the heat transfer made through the gases is sufficient and low resistance is incurred.
Moving on from the GDL the gases then make their way to the PEM. The PEM has been coated with a catalyst most often Pt is used. At the anode, the hydrogen molecule is caused to split forcing the electron to flow out. The PEM is a semi-permeable polymer membrane which only allows the positive ions to be conducted to the cathode.
The electrons which have been previously split at the anode rejoin at the cathode producing heat and water. The water makes its way out of the cathode along with excess nitrogen and oxygen.
The water usually produced may cause flooding and may impact the reactant transportation. This is why it is ensured that the temperature of the Fuel cell does not go beyond 80 degrees Celsius.
Anode reaction:
2H2 → 4H+ + 4e−
Cathode reaction:
O2 + 4H+ + 4e− → 2H2O
Overall cell reaction:
2H2 + O2 → 2H2O + heat + electrical energy
The energy obtained from the reaction is 1.23 V
Analyzing the Fuel Cell
Let us briefly analyze the PEMFC. Some of the advantages of the PEMFC are as follows.
- High efficiency
- Highly reliable
- There is a great decrease in Noise Pollution
- Environment friendly-A great alternative to fossil fuels
- Compact size
Disadvantages of the Fuel Cell
- High Production Cost
- Infrastructure not available to manage hydrogen
- Technology still in the prototype stage
- Hydrogen is yet hard to produce
- High sensitivity
PEM Assembly in Electrolysis
We also use Polymer electrolyte membranes in the process of electrolysis. PEM water electrolysis is an intricate process we will explain further. Although there are other ways for hydrogen extraction PEM electrolysis happens to be the most promising. It has a higher productivity and efficiency rate along with a compact design.
The design of The PEM Electrolyzer manages to overcome the problems that take place during a typical alkaline electrolyzer. It helps magnificently in the areas of partial load and hydrogen density. It provides fast dynamic response times and facilitates high gas purities.
You can use the hydrogen that you obtain from PEM electrolysis in hydrogen fueling stations as well as household units. PEM electrolysis actually helps to increase the operating pressure at which you store the hydrogen in vessels. Thus, this prospect is mind-blowing for stationary systems that have a relatively small power capacity.
Most interestingly PEM electrolysis provides hydrogen that can also use form biogas or flue gases which are excellent sources of renewable energy.
The biggest advantage of PEM electrolysis is its ability to perform at high densities.
The Fundamentals of PEM Electrolysis
The whole process is endothermic and so electricity is applied as the energy source. The PEM electrolysis is very much similar to the PEM fuel cell. The PEM works with a very thin membrane measuring about 2mm in thickness. Due to its solid structure, there is a low gas crossover that enables high products of gas purity.
Reactions
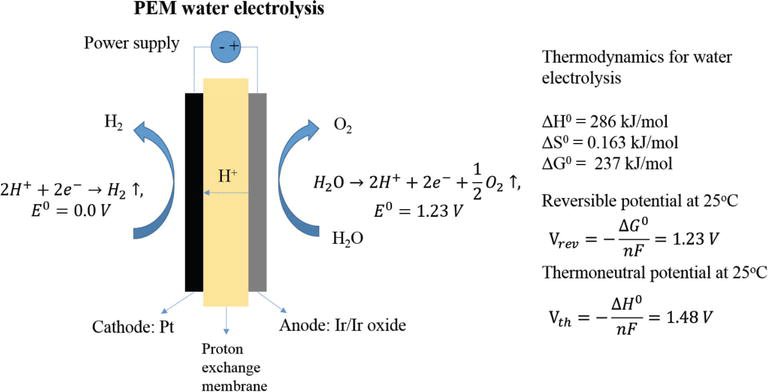
The reaction that takes place at the anode is popular by the name Oxygen Evolution Reaction.
Anode Reaction
At the anode, oxidize the water to produce oxygen, electrons, and protons. Then transfer the protons over the PEM so that you can reduce them to hydrogen. The catalyst typically used for this reaction is iridium. It is popular due to its anti-corrosive properties.
Cathode Reaction
Hydrogen Evolution Reaction is the name of the reaction that takes place here. The cathode reaction is very similar to the PEM Fuel cell. Manufacturers use a Piece of carbon paper to make the gas diffusion layer.
So, to conduct the protons, you use the membrane to produce hydrogen. The figure below will give you a better insight as to the reactions occurring at the cathode and anode.
Efficiency
To measure the efficiency of PEM electrolysis, use the higher heat value. Thus, the temperature for operation in PEM Electrolysis is 80 degrees Celsius. Therefore it is possible to redirect the waste heat to make the steam. This increases efficiency immensely.
The calculated value of efficiency for PEM electrolysis is 80 percent. This has been calculated in terms of the hydrogen produced as per the unit of electricity required for the reaction. We anticipate that efficiency will increase with the passage of time.
PEM Assembly Producers
PEM assembly is a much-sought process and currently there are many producers. It is essential that a good PEM has good proton conductivity, mechanical durability, and high efficiency. Nafion is the first PEM that fell in line with all the requirements.
There are many other companies which are producing PEM but most of them are based on the Nafion polymer structure. To ensure that the chemical and thermal stability is optimum, use the Carbon-fluorine resin. So, Nafion makes use of the sulfonic group which in case of water absorption can make good mobility.
Recently research has to lead to reduce the poisoning of methanol on the platinum catalyst. This can only be done if an original PEM is used or by doping the polymer to reduce the swelling of the Nafion.
Conclusion of PEM Assembly
Proton Exchange Membrane technology is an outstanding breakthrough in the modern world scenario. It has become essential for future climate-friendly and efficient devices. Below we will be giving you a comprehensive introduction to the fascinating PEM Assembly methodology used in applications.
This was a brief introduction to the PEM Assembly and its incredible uses. Hopefully, you have a better insight. We offer a wide range of PEM services. To learn more about our services, we recommend you reach out to us.
Our learned staff would provide you with all the information. They would ensure that you are able to find the best product.




